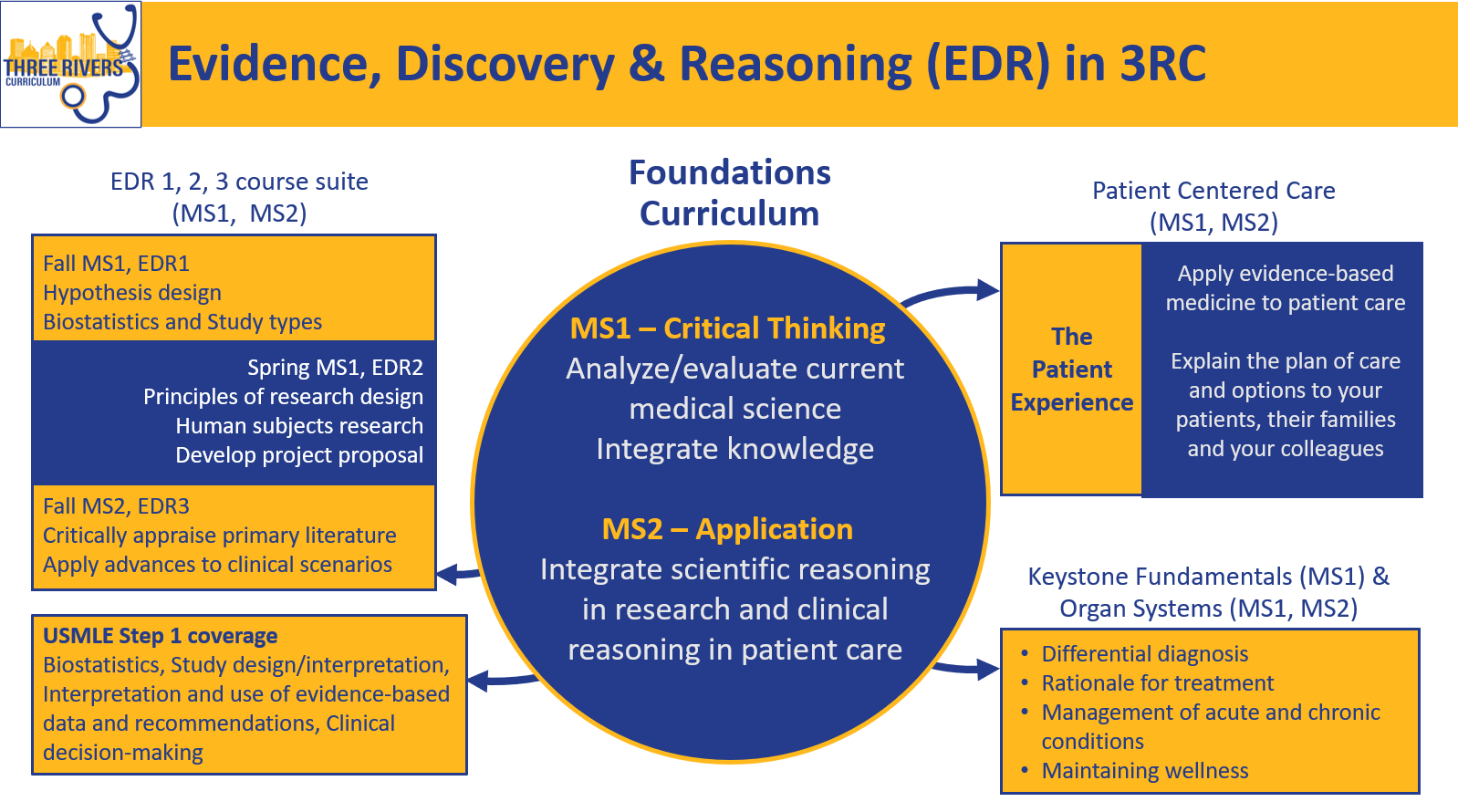
Course Description
The skills of reading biomedical literature, interpreting data, and evaluating clinical studies are the focus of this course, integrating themes of clinical expertise and scientific reasoning. Students learn basic statistical methods and how clinical trials, medical databases, and translational medicine are foundations of evidence-based medicine and patient-centered care. Together, the EDR1-3 course suite ensures a progressive build in student knowledge, and reinforces past skills learned as students progress through the course sequence.
USMLE Step 1 Content Area: Biostatistics, Study design & interpretation, Interpretation and use of evidence-based data and recommendations in clinical decision-making
Course Objectives
- Design and create a PICOT-structured or clinical question to search the biomedical research literature for results that inform a patient scenario
- Describe and identify potential study designs and associated benefits and limitations
- Summarize statistical testing foundational concepts and criteria for choosing a specific statistical test
- Correctly interpret statistical significance from the output of statistical tests including test statistics, p-values, and confidence intervals
- Calculate, report, and interpret study outcomes including odds ratios, relative risk, absolute risk reduction and NNT/H including statistical and clinical significance
- Identify bias and confounding in case prompts and studies including sources of bias, types or bias and way to limit both bias and confounding
- Correctly interpret survival data including curves and hazard ratios
- Define components of trial execution with specific attention to allocation, blinding, and sampling
- Compare types of outcome measures including benefit and drawbacks of each type
- Calculate diagnostic test characteristics and apply to clinical decisions in proposed cases
- Evaluate and interpret meta-analysis including quality of the analysis and interpretation of forest plots
- Interpret and calculate cost analysis data including common components such as cost-effectiveness ratio, incremental cost-effectiveness, and utility factors
- Search the literature to find articles pertinent to a patient.
- Critique the medical literature and relate it to a patient. Analyze and evaluate a biomedical research article—background, hypothesis, study design, results, statistics, key information, validity of conclusions in the setting of the patient.
- Identify and correct inaccuracies of Large Language Model (LLM) assessments of the research article appraised. Provide evidence and reasoning from primary literature for the corrections.
- Develop an organized approach to explaining clinical and translational research to patients.
- Engage in self-directed learning and meta-cognition in research and medicine.
Educational Methods
- Small group workshops
- Self-study
- EDR1: Weekly quizzes
- EDR2&3: Listening and providing feedback to peers; Presentations
Assessment
EDR1: Assessment for this course is based on a cumulative score of >=70% on the weekly quizzes
EDR2&3: Assessment is based on active participation, presentations, written research proposal (EDR2), and written critical appraisal of scientific article (EDR3).
Requests for excused absences should be submitted via Elentra. Unexcused absences may result in grading penalties as outlined in the Policy on Absence and Attendance.
